√99以上 heterogeneous mixture composed of polar and nonpolar solutions 335612
Composed of a solute and a solventStart studying Solutions Learn vocabulary, terms, and more with flashcards, games, and other study toolsSaying that describes how polar and nonpolar solutions work Suspension heterogeneous mixture with large particles that are evenly dispersed, but settle into layers when left to stand
Heterogeneous Compounds Clue Chemistry Life The Universe And Everything
Heterogeneous mixture composed of polar and nonpolar solutions
Heterogeneous mixture composed of polar and nonpolar solutions-Classifications of Mixtures • Heterogeneous Mixtures—composed of different types of phases of substances ex Fruit salad Granite • Homogeneous Mixtures—the same throughout (substances have dissolved in one another) ex Salt water Alloys (metal mixtures) Solutions are • • A homogeneous 1 mixture of two or more substances in a A heterogeneous mixture is a mixture that is in at least two different states of matter (two phases), its components are mixed in a nonuniform way and it is possible to differentiate them with the naked eye While in a homogeneous mixture the components are distributed in the same way, in any region of the mixture, in a heterogeneous mixture




Chemistry Ii Water And Organic Molecules
Figure 1 (a) A solution is a homogeneous mixture that appears clear, such as the saltwater in this aquarium (b) In a colloid, such as milk, the particles are much larger but remain dispersed and do not settle (c) A suspension, such as mud, is a heterogeneous mixture of suspended particles that appears cloudy and in which the particles canThe interesting heterogeneous structure of the NMA−LA DES provides a molecular landscape with different features that could accommodate different solutes with preferential affinity to the particular domains (nonpolar or polar) in the mixture This work is aimed at characterizing the location of different solutes within the•polar solute polar solvent = soluble •nonpolar solute nonpolar solvent = soluble •nonpolar solute polar solvent = not soluble •polar solute nonpolar solvent = not soluble For example, water is a polar substance and oil is nonpolar Water and oil are not soluble in one another As a result, they don't mix and instead
The mixture is heterogeneous and can be separated by filtration (D)The mixture is heterogeneous and cannot be separated by filtration 6 The phrase "like dissolves like" refers to the fact that _____ (A) gases can only dissolve other gases (B) polar solvents dissolve polar solutes and nonpolar solvents dissolve nonpolar solutesNonpolar solutes polar solvents polar solutes nonpolar solvents polar solvents from CH 104 at Portland Community College Browse Textbook Solutions A _____ is composed of visibly distinguishable parts and does not have uniform composition homogeneous mixture heterogeneous mixture 4 _____ is an example of a homogeneous mixture So, NaCl—a very polar substance because it is composed of ions—dissolves in water, which is very polar, but not in oil, which is generally nonpolar Nonpolar wax dissolves in nonpolar hexane, but not in polar water Figure \(\PageIndex{2}\) Water (clear liquid) and oil (yellow) do not form liquid solutions (CC BYSA 10 Generic;
Mixture Definition Compounds are those substances that are composed of many identical molecules and consist of two or more atoms from more than one element linked together by chemical bonds Mixtures are substances made up of two or more different substances that are not chemically combined and do not undergo chemical change CompositionThis video explains about the concept of SolutionMixture and its types ( Homogeneous and Heterogeneous Mixture ), Components of Solution Solute and SolvenA mixture in which components are not uniformly mixed suspension a heterogeneous mixture in which solutelike particles settle out of the solvent, like sand in water




Mixtures



Difference Between Solvent And Solute Definition Properties Examples
For a solution to form, the solvent and solute molecules must be attracted to each other "Like Dissolves Like" Polar solvents dissolve polar solutes Nonpolar substances are attracted to other nonpolar substances, therefore nonpolar solvents will dissolve nonpolar solutes Examples I 2, Hexane, Cooking Oils Like dissolves like Slide 1 Solutions What is a Solution? The types of compounds that should be drawn in the reactant mixture How much of each element is present in the product mixture Step 1) This question introduces the reaction and tells us that the two reactants are NO and O2 Step 2) If you count how many atoms of each element are present, you would get 8 N atoms and 12 O atoms




Heterogeneous Compounds Clue Chemistry Life The Universe And Everything




Solubility And Polarity C11 4 5
The ionic end is attracted by water (polar), illustrated in Figure \(\PageIndex{5}\) As a result, the soap or detergent molecules become oriented at the interface between the dirt particles and the water so they act as a kind of bridge between two different kinds of matter, nonpolar and polarA nonelectrolyte is composed of _____in solution answer choices molecules molecules and ions ions none of the above nonpolar solutes dissolve in polar solvents s Question 19 SURVEY heterogeneous mixture solution compound alternativesBecause homogeneity is part of the definition of a solution If it's not homogenous it's a suspension or some flavor of colloid (a foam, emulsion, or sol if the dispersion medium is a liquid) Solutions exist when it's energetically more favorable



Difference Between Solvent And Solute Definition Properties Examples




Ch 13 Solutions Flashcards Quizlet
Single standard stock solutions of the analytes (with concentrations ranging from 1731 to mg L −1) and the ISs (concentration between 10 and 296 mg L −1) were prepared by exact weighing of powder or liquid and dissolution in acetone (nonpolar pesticides) or methanol (MeOH) (polar pesticides), and stored in a freezer ( °C)Suspensions, colloids and solutions A suspension is a heterogenous mixture containing large particles that will settle on standing Sand in water is an example of a suspension Is water a colloid?A heterogeneous mixture is composed of visibly indistinguishable this is a glass container with a stopcock on the bottom Two immiscible solutions are put in it Since the solutions do (Boiling Point = 125 C) C Solid I (nonpolar solid) and NaCl D The mixture of inks present in a "Sharpie" permanent marking pen E Nickel shavings and




Solutions Solutions Homogeneous Mixture Of Two Or More Substances Consist Of A Solute And A Solvent Properties Of A Solution Solutions Have Variable Ppt Video Online Download
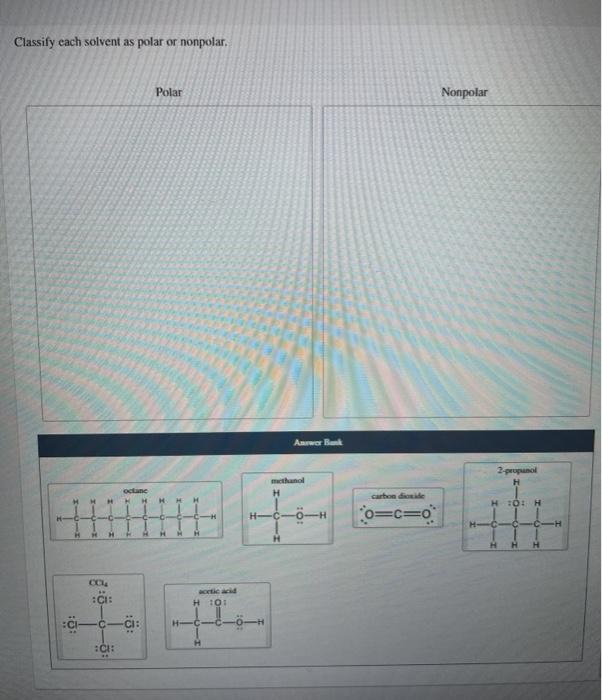



Classify The Given Mixtures As Heterogeneous Or Chegg Com
• Heterogeneous – mixtures that do not A solution is a mixture that appears as a single substance but is composed of particles of two or more substances that other is nonpolar (oil) Ionic and polar solutes will only dissolve in ionic or polar solvents like water NonpolarMixtures of nonpolar molecules 37 Mixtures containing rigid linear molecules The experimental work of Patterson and his coworkers mentioned in the previous section has concentrated on mixtures of aliphatic and alicyclic hydrocarbons where the pseudolinear molecules used, typically the n alkanes, possess considerable degrees of flexibilityPolar and Nonpolar Solvents • A liquid composed of polar molecules is a polar solvent Water is a polar solvent • A liquid composed of nonpolar molecules is a nonpolar solvent Hexane is a nonpolar solvent Solutions • Concentration A measure of the amount of solute dissolved in solution Concentration = Solute Solution Grams, molecules
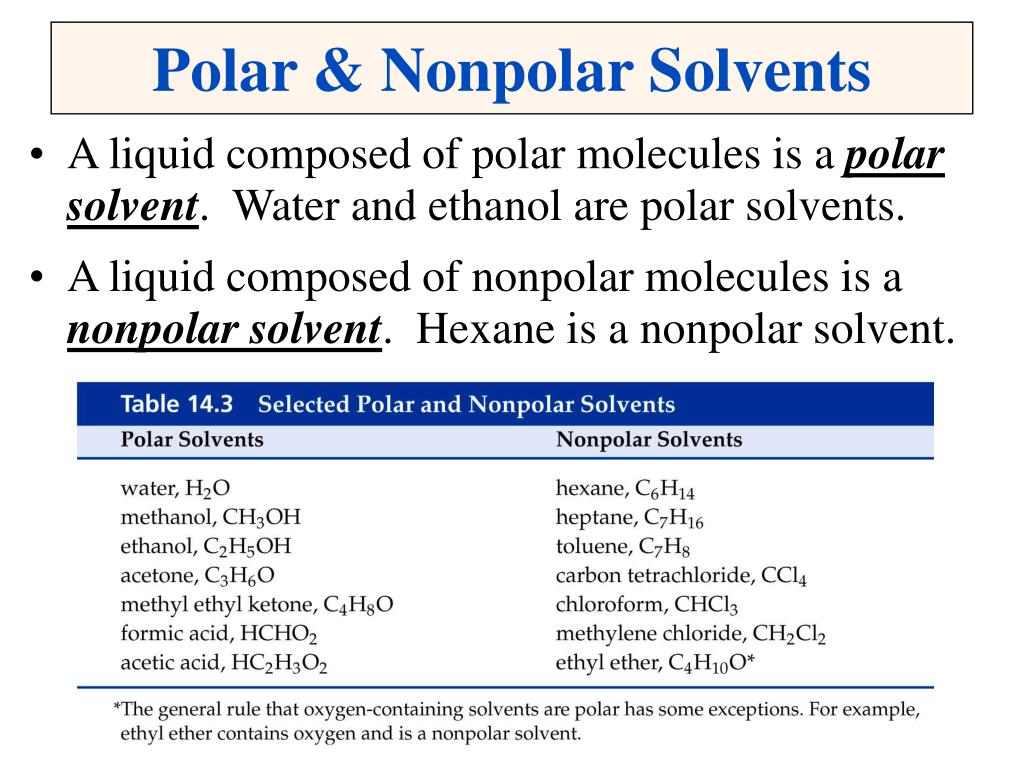



Ppt Solutions Powerpoint Presentation Free Download Id




Is A Mixture Of Water And Oil Homogenous Or Heterogeneous Quora
MrPemble Chemistry B Period 3 Names Melanie Mondragon Space Sand Lab Introduction Polar vs NonPolar Atoms are composed of protons, neutrons, and electrons Two or more atoms bond together to create a molecule by sharing electrons If the electrons are distributed evenly throughout the molecule, it creates a nonpolar molecule If the protons and electrons are notThe solution structure is found to be dominated by the cosolvent clustering at high IL concentrations, and segregation of the IL into polar and nonpolar domains in dilute IL solutionsWhereas Nonpolar solutes dissolve in nonpolar solvents Solvents that are composed of polar molecules include water and dissolves the other molecules such as table salt non polar solvents such as gasoline dissolves non polar substances such as wax Ex Intermolecular forces Water/SaltP Water/SugarP Water/OilPN Isopropyl Alcohol / SaltP Isopropyl Alcohol / SugarP




Tailored Media For Homogeneous Cellulose Chemistry Ionic Liquid Co Solvent Mixtures Gericke 11 Macromolecular Materials And Engineering Wiley Online Library



The Solution Process
The mixture of coffee and milk would constitute a homogeneous mixture This is because when the two substances blend together, the mixture itself takes one "same" (homogeneous) form Homogeneous mixtures are also known as solutions, which are mostly composed of liquids (including vinegar), but can include gases emulsions are colloids, heterogeneous mixtures composed of tiny particles suspended in another immiscible (unmixable) material Since oil is nonpolar, and vinegar is a polar aqueous solution, the two do not(a) A solution is a homogeneous mixture that appears clear, such as the saltwater in this aquarium (b) In a colloid, such as milk, the particles are much larger but remain dispersed and do not settle (c) A suspension, such as mud, is a heterogeneous mixture of suspended particles that appears cloudy and in which the particles can settle




Chapter 7 Solutions A Solution Is A Homogeneous Mixture That Consists Of The Solute And The Solvent Ppt Video Online Download
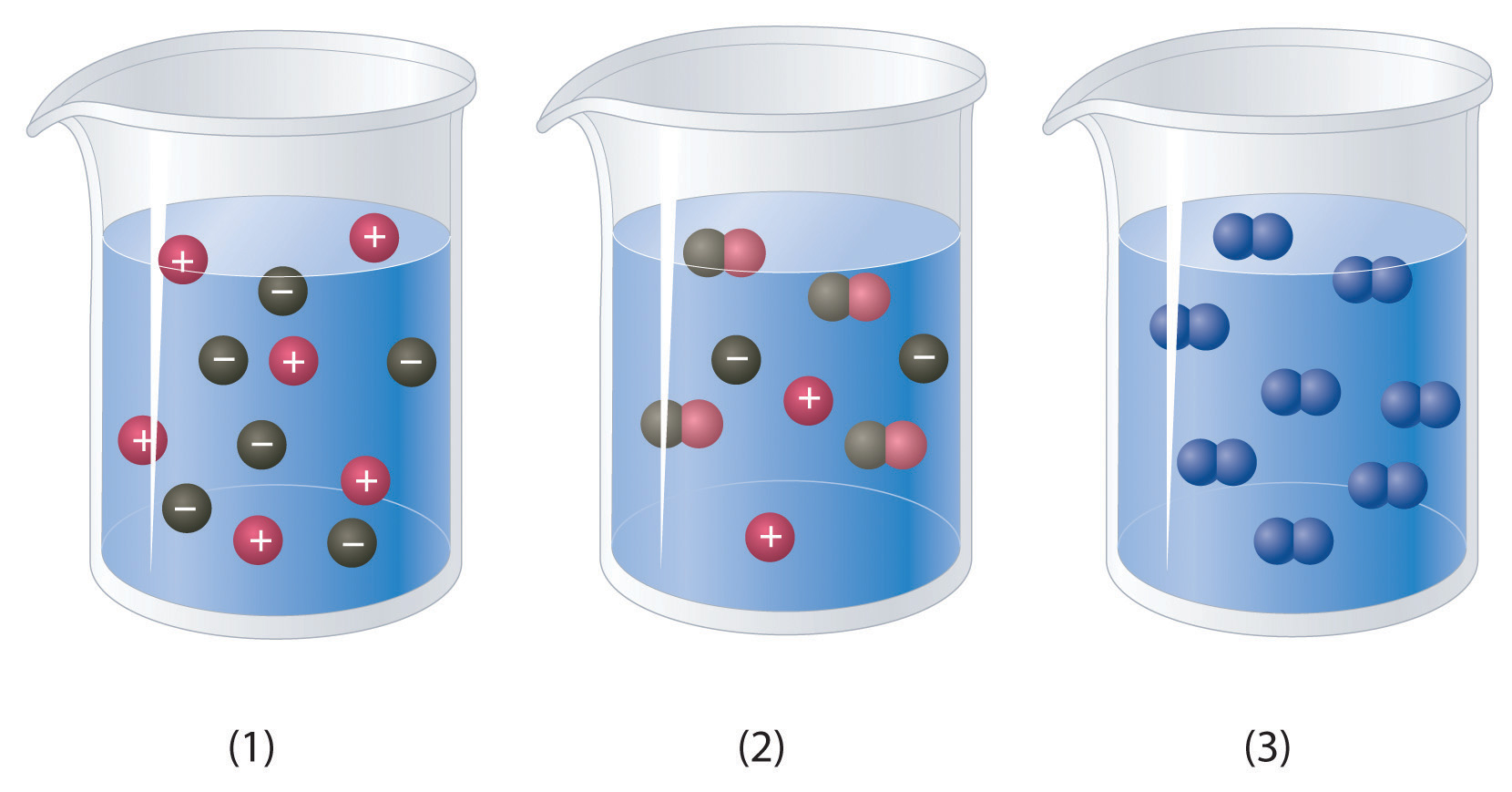



Ch104 Chapter 7 Solutions Chemistry
Main Difference – Polar vs Nonpolar Molecules Atoms of different or same elements come together to form molecules The bond which is formed by sharing a pair of electrons between two atoms is called a "Covalent Bond" Different atoms show attraction toColloids are generally considered heterogeneous mixtures, but have some qualities of homogeneous mixtures as well(a) A solution is a homogeneous mixture that appears clear, such as the saltwater in this aquarium (b) In a colloid, such as milk, the particles are much larger but remain dispersed and do not settle (c) A suspension, such as mud, is a heterogeneous mixture of suspended particles that appears cloudy and in which the particles can settle



Http Employees Oneonta Edu Viningwj Chem112 Chapter 13 Solutions And Other Mixtures Pdf




Chemistry Ii Water And Organic Molecules
A heterogeneous mixture is the type of mixture in which the composition of the solute is not uniform throughout the mixture Thus, in a heterogeneous mixture, all parts of the mixture do not have the same concentration throughout The components of a heterogeneous mixture are easily visible in the mixture as the size of the particles tends to be largerA solution is simply a homogeneous mixture A solution consists of the substance that is referred to as the solute being mixed into another substance, called the solvent When salt is mixed with water, the salt is the solute and the water is the solvent The components of solutions are either atoms, molecules, or ions Skin is a heterogeneous mixture, composed of many different cells, which are composed of many different compounds Polarity is decided by the configuration of the molecule Because it is made of
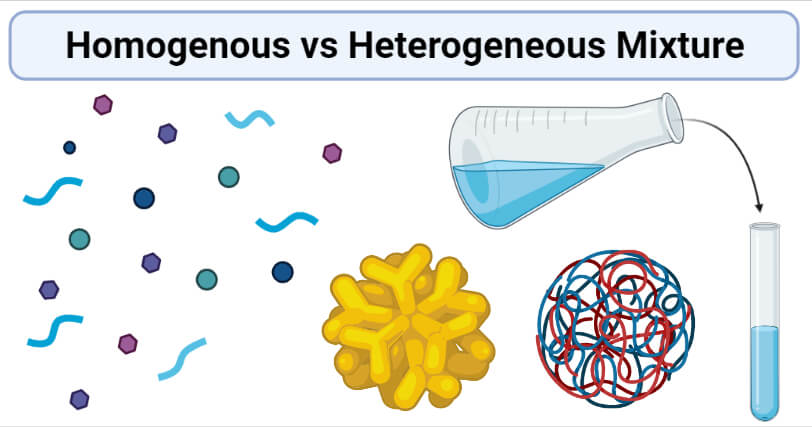



Homogenous Vs Heterogeneous Mixture Definition 8 Key Differences Examples




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii
Start studying Physical science chapter 7solutions Learn vocabulary, terms, and more with flashcards, games, and other study toolsSuppose we have a molecule that is very polar on one end, but nonpolar at the other Soap, for instance, is an ionic compound, but while the cation is usually just a sodium ion, the anion is more complicated This molecular anion most often contains a very polar "carboxylate group", composed of a carbon with two attached oxygens A cup of coffee is definitely a solution, in the sense that it is composed of dissolved solutes Is milk and coffee a solution?
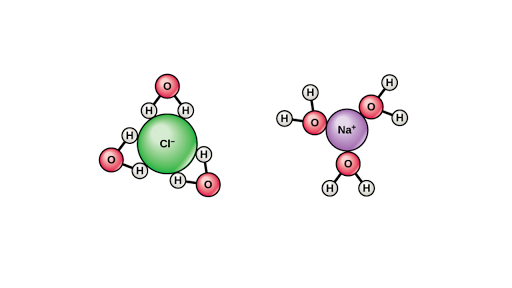



Solvent Properties Of Water Article Khan Academy




Solutions Solutions Are A Homogeneous Mixture Of Two Or More Substances In A Single Phase Composed Of 1 Solvent The Substance That Does The Dissolving Ppt Download
For a solution to form, the solvent and solute molecules must be attracted to each other "Like Dissolves Like" Polar solvents dissolve polar solutes Nonpolar substances are attracted to other nonpolar substances, therefore nonpolar solvents will dissolve nonpolar solutes Examples I 2, Hexane, Cooking OilsSee the answer ___1) A Solution is a) A heterogeneous mixture whose components can be combined in any proportion b) A homogeneous mixture whose combined components react with each other c) A heterogeneous mixture whose components retain their chemical and physical properties d) A homogeneous mixture whose components are evenly distributed(a) A solution is a homogeneous mixture that appears clear, such as the saltwater in this aquarium (b) In a colloid, such as milk, the particles are much larger but remain dispersed and do not settle (c) A suspension, such as mud, is a heterogeneous mixture of suspended particles that appears cloudy and in which the particles can settle




Properties Of Solution Solution Defined Homogeneous Mixture Composed




10 Heterogeneous And Homogeneous Mixtures
Classify the given mixtures as heterogeneous or homogeneous Heterogeneous Homogeneous tomato juice a bowl of chicken noodle soup a cup of tea with honey urine Pepto Bismol gasoline whole blood Answer Bank incorrect For mixtures composed of solid particles in a liquid, the level of transparency can be a predictor of the type of mixtureThe ionic end is attracted by water (polar), illustrated in Figure \(\PageIndex{5}\) As a result, the soap or detergent molecules become oriented at the interface between the dirt particles and the water so they act as a kind of bridge between two different kinds of matter, nonpolar and polarHeterogeneous MIXTURES MIXTURES Solutions (All gaseous mixtures are solutions) solid in gas (soot in air) liquid in gas (humid air) gas in gas (air) Soapis another molecule with a nonpolar end and a polar end that is good at lifting oils and other nonpolar solutes in water which is polar




Solubility Word Search Wordmint




Ppt Solutions Powerpoint Presentation Free Download Id
A homogeneous mixture is composed of visibly distinguishable parts and does not have uniform composition A heterogeneous mixture is composed of visibly indistinguishable parts which have a uniform composition True False 4 A _____ dissolves into a _____Homogeneous mixture of a solvent and a solute Homogeneous Mixture Same throughout Not filterable *No boundaries can be detected inA homogeneous mixture with very small particles that do not settle out of the solution;




Solution Chemistry Wikipedia




Beginner S Guide To Convergence Chromatography 2 Waters
Mixtures by Ron Kurtus (revised 13 January 06) A mixture is the blending of two or more dissimilar substances A major characteristic of mixtures is that the materials do not chemically combine Mixtures can be divided into those that are evenly distributed (homogeneous) and those that aren't (heterogeneous)The types of mixtures are a suspension, colloid or solution




Solutions Advanced Ck 12 Foundation




Chapter 2 Module 2 Section 2 03 2 04 Flashcards Quizlet
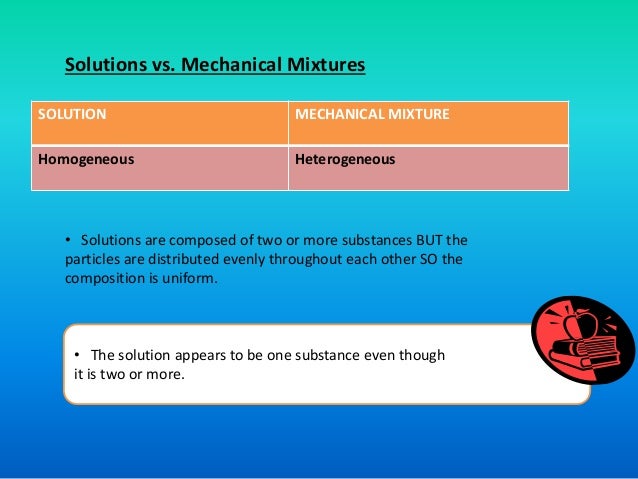



The Nature Of Solution




Section 9 Lecture Notes Ch 9 Solutions Solution Is Another Name For Homogeneous Mixture Mixture As Material Composed Of Two Or More Substances In Solution Studocu



Http Www Webassign Net Question Assets Wertzcams3 Ch 10 Manual Pdf



Solution Is A Homogeneous Mixture Consists Of Solvent And Solute S Aqueous Solution Has Water As Solvent Docslib



Solutions




Mixtures And Solutions 14 1 14 2 Big Idea Manualzz



Homogeneous And Heterogeneous Mixtures Springerlink



The Solution Process




Essentials Of General Organic And Biochemistry By Macmillan International Higher Education Issuu




Choose The Incorrect Statement A A Homogeneous Mixture Of Clutch Prep



How Is Molecular Polarity Related To Solubility Socratic
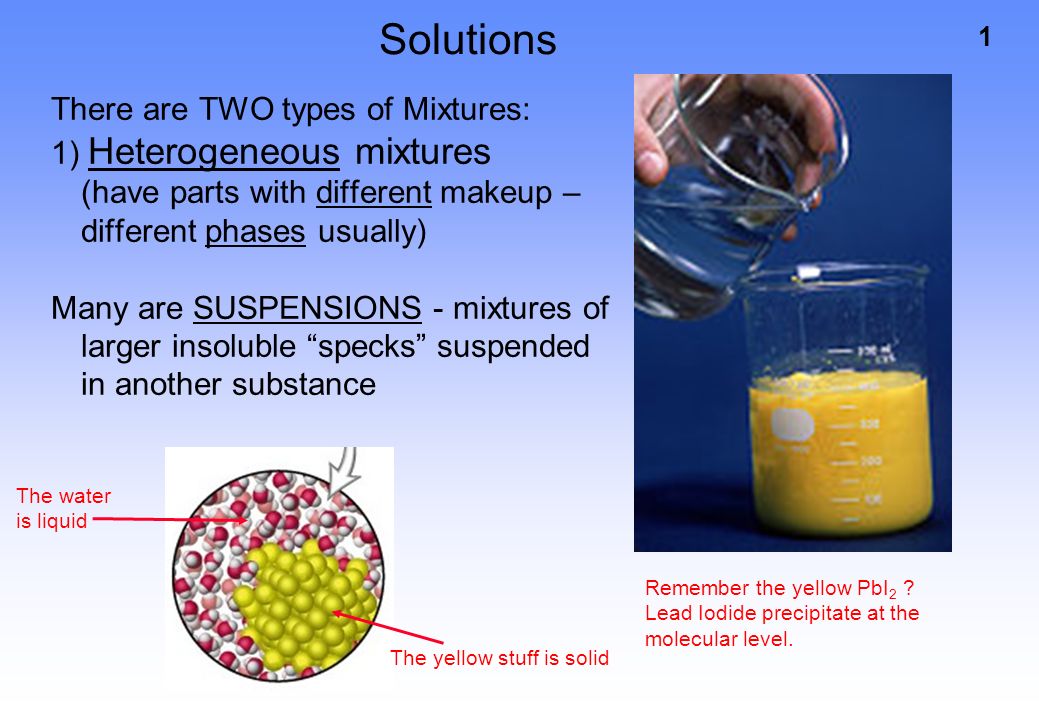



1 Solutions There Are Two Types Of Mixtures 1 Heterogeneous Mixtures Have Parts With Different Makeup Different Phases Usually Many Are Suspensions Ppt Download



Mixtures Solutions And Suspensions Tmjh 8th Grade Science



Note Packet



What Is The Difference Between A Substance And Its Solvent Quora



1




11 1 The Dissolution Process Chemistry




Properties Of Solution Solution Defined Homogeneous Mixture Composed




Homogeneous Vs Heterogeneous Mixtures A Comparison Expii



1
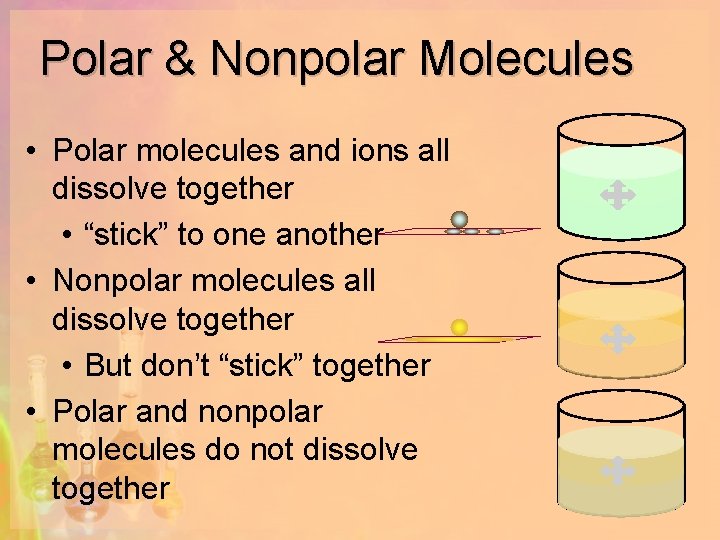



Solutions What Is A Solution Homogeneous Mixture Of




Solutions And Mixtures Bingo Cards Wordmint




Solutions Solution Homogeneous Mixture Made Up Of Very Small Particles The Size Of Individual Molecules Atoms Or Ions Parts Of A Solution Solute Ppt Video Online Download
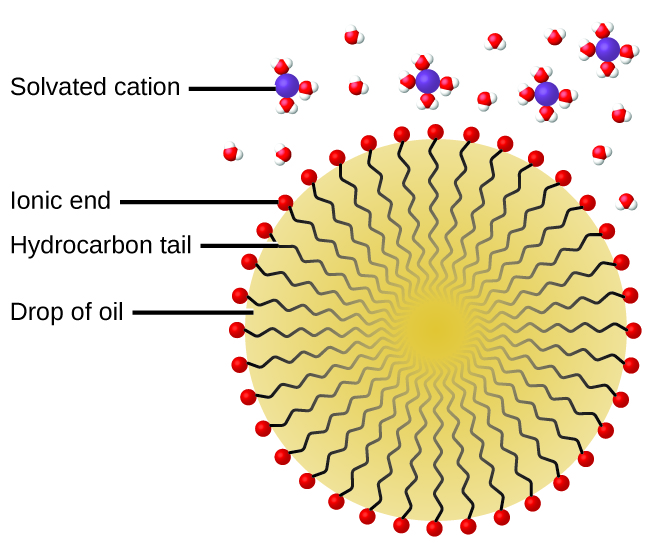



Colloids Chemistry Atoms First 2e




Types Of Mixtures Intermolecular Forces And Properties Ap Chemistry Khan Academy Youtube




13 Best Examples Of Heterogeneous Mixtures Rankred




Liquid Liquid Solutions Chemistry For Non Majors
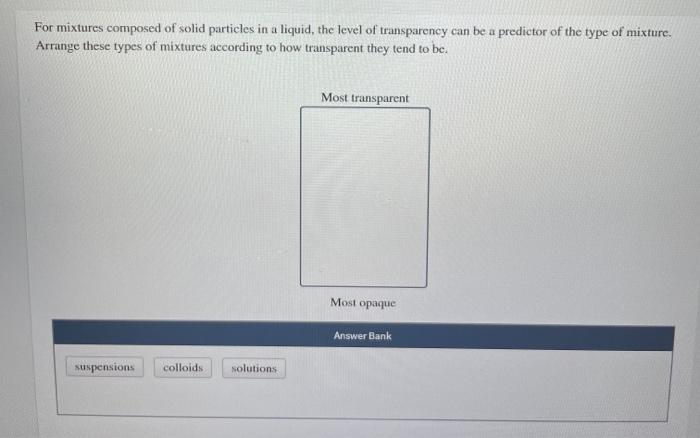



Classify The Given Mixtures As Heterogeneous Or Chegg Com




What Is A Solution Overview Examples Expii




Ppt Solutions Powerpoint Presentation Free Download Id 1




Lesson 1 Pure Substances And Mixtures Heterogeneous And Homogeneous Pdf Free Download



Molecules Free Full Text Vegetable Oils As Alternative Solvents For Green Oleo Extraction Purification And Formulation Of Food And Natural Products Html



Http Www Tandfonline Com Doi Pdf 10 1080




8 1 Mixtures A Solutions A Solution Is A Homogeneous Mixture That Contains Small Particles Liquid Solutions Are Transparent Solutions Consist Of Two Ppt Download



3



Http Www Austincc Edu Mohan Documents 14 Lecture Pdf
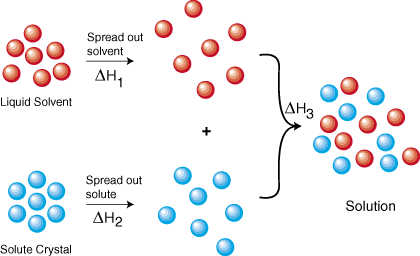



The Solution Process
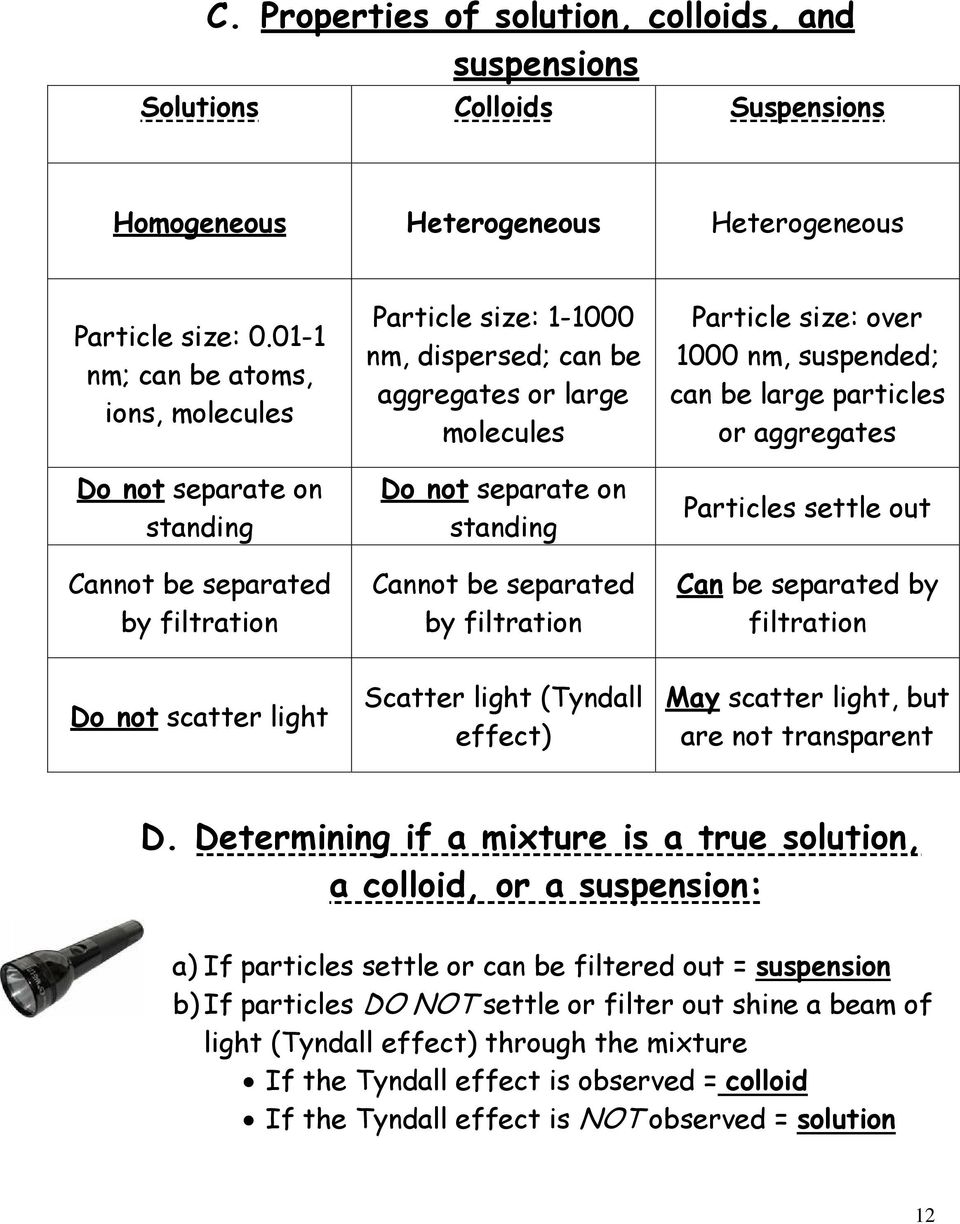



A Types Of Mixtures Pdf Free Download




Pdf Chem 14 001 Mixtures True Solutions Prince Tan Academia Edu




Chapter 14 Class Notes
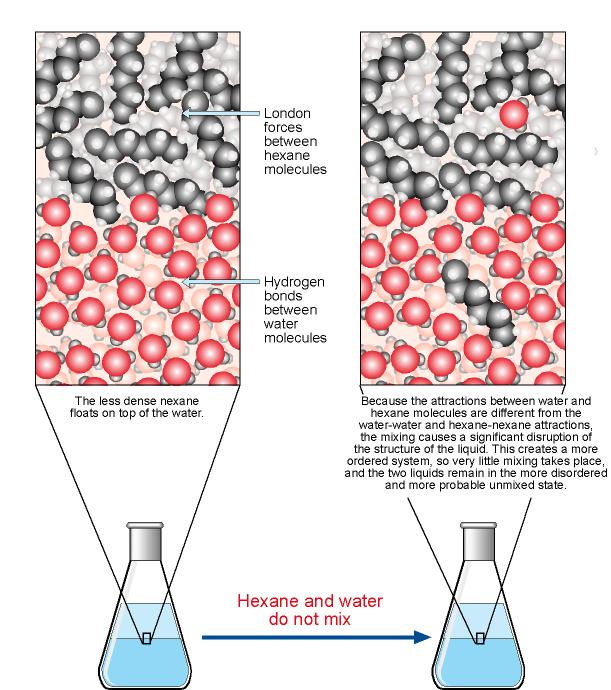



The Solution Process




13 2 Solutions Homogeneous Mixtures Chemistry Libretexts




2 1 Homogeneous And Heterogeneous Mixtures




Solubility Protocol




What Do You Need To Know About Heterogeneous And Homogeneous Mixtures



Colloids Chemistry Atoms First 2e




Atoms And Mixtures




Which Of These Mixtures Are Heterogeneous Select All That Apply A Oil And Water B Salt And Water C Brass An Alloy Of Copper And Zinc D Granite A Type Of Rock



Ch104 Chapter 7 Solutions Chemistry




Colloids Chemistry



1




Pure Substances And Mixtures 1 Pure And Mixed




Chapter 9 Molecules And Ions In Solutions Studocu




Properties Of Solution Solution Defined Homogeneous Mixture Composed




1 8 1 Solutions Mixture 2 Or More Components Solute Substance In The Lesser Amount Solvent Substance In The Greater Amount Solution Homogeneous Mixture Pptx Powerpoint




Chapter 18 Intro To Bio Chem



Solutions
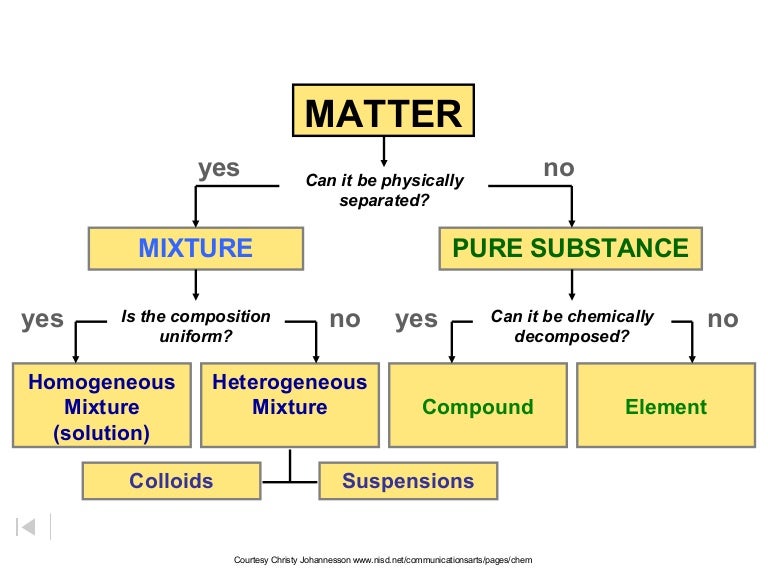



Ch 2 Classification Of Matter Ppt




The Solution Process




Ch2 Dissolution An Energy Phenomenon Flashcards Quizlet




11 5 Colloids Chemistry




11 1 The Dissolution Process General College Chemistry Ii
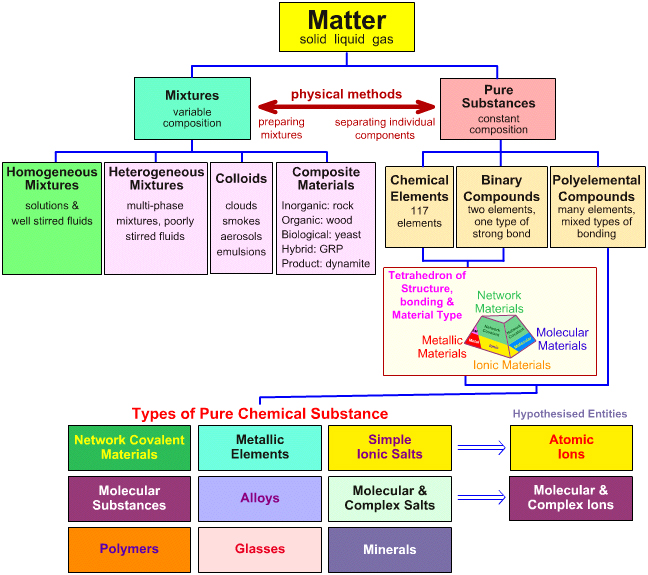



Matter Chemogenesis




Classifications Of Mixtures Heterogeneous Mixtures Composed Of Different Types Of Phases Of Substances Ex Fruit Salad Granite Homogeneous Mixtures The Ppt Download
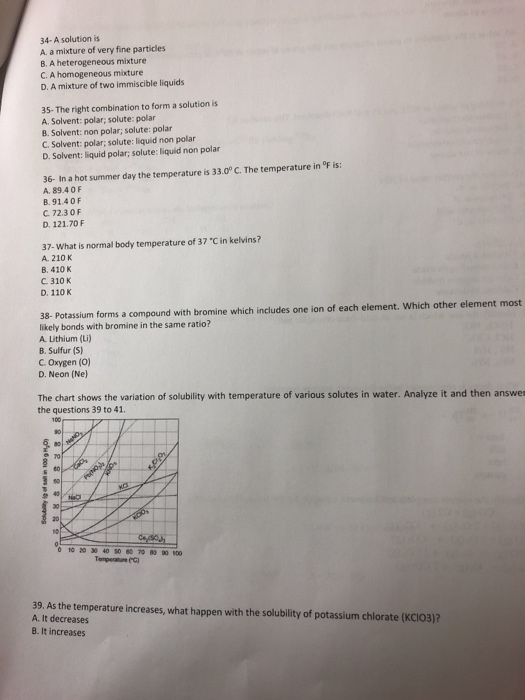



34 A Solution Is A A Mixture Of Very Fine Particles Chegg Com




Mixtures




The Nature Of Solution



Separate Liquids With Salt Scientific American
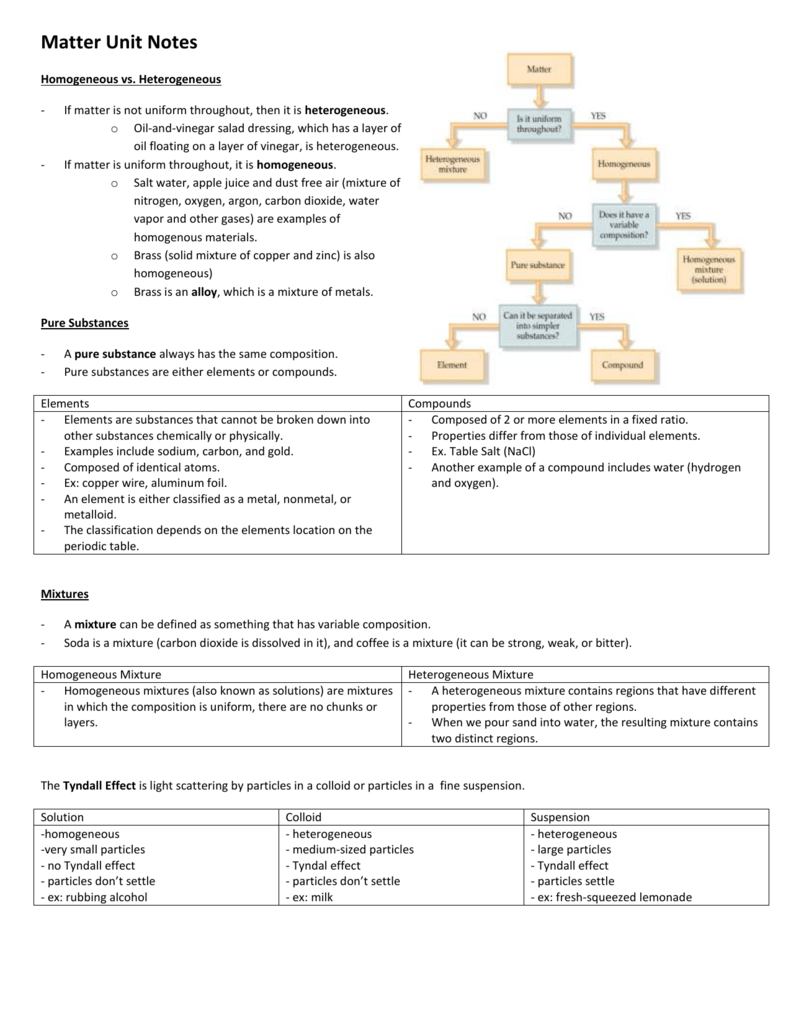



Matter Unit Notes
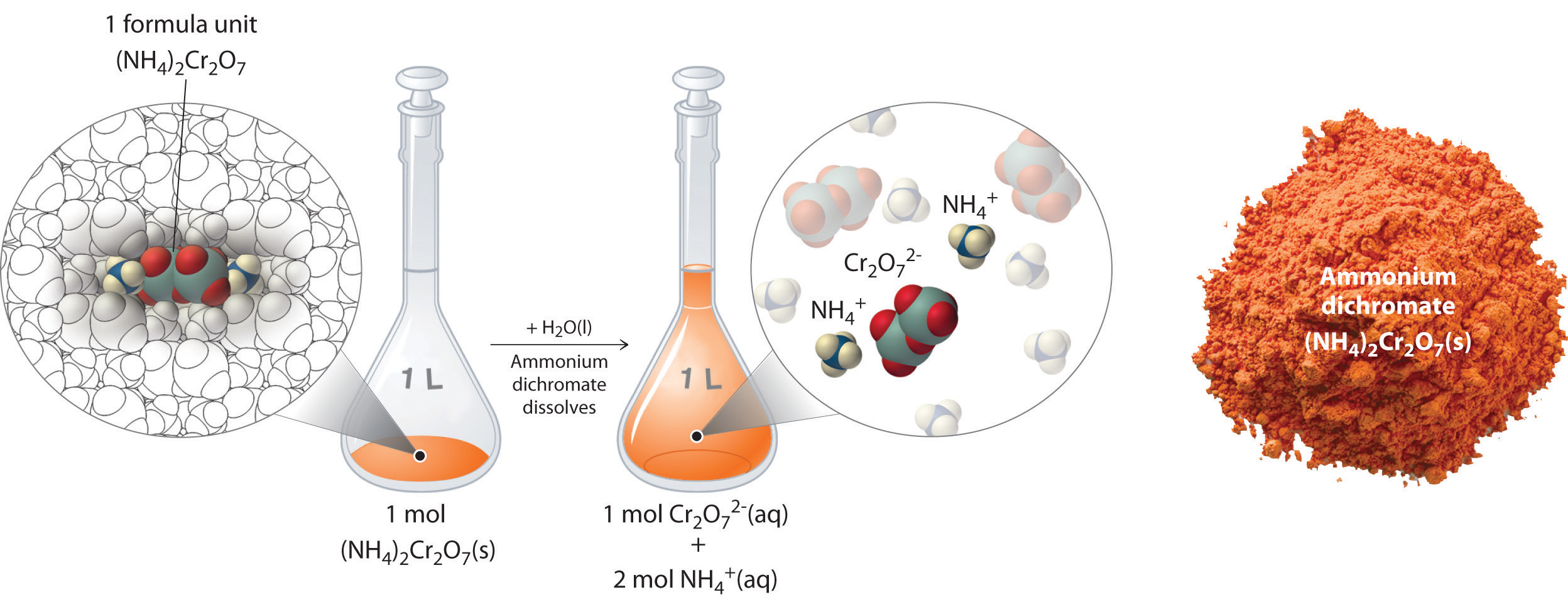



Ch104 Chapter 7 Solutions Chemistry
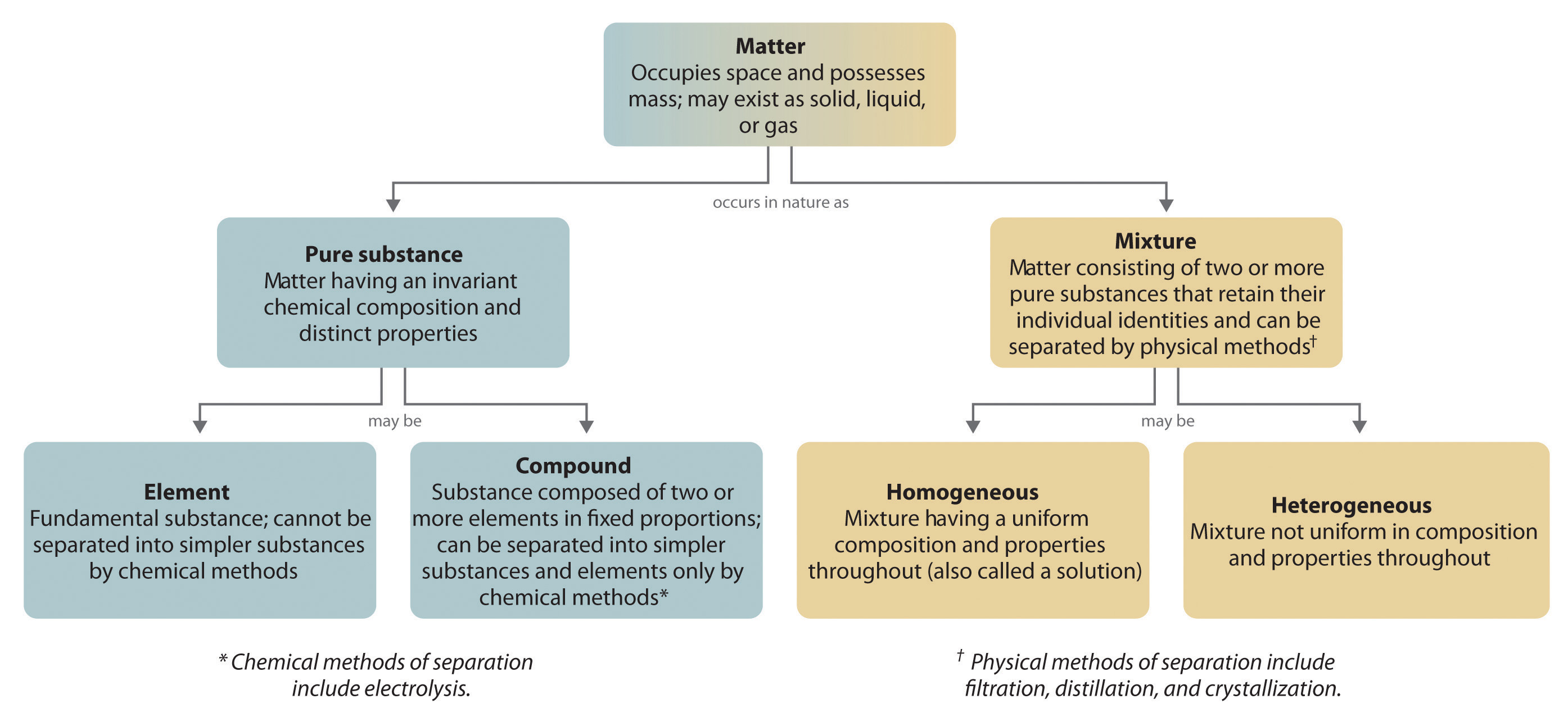



Introduction To Chemistry



Piezoelectricity And Rotostriction Through Polar And Non Polar Coupled Instabilities In Bismuth Based Piezoceramics Scientific Reports




Properties Of Solution Solution Defined Homogeneous Mixture Composed
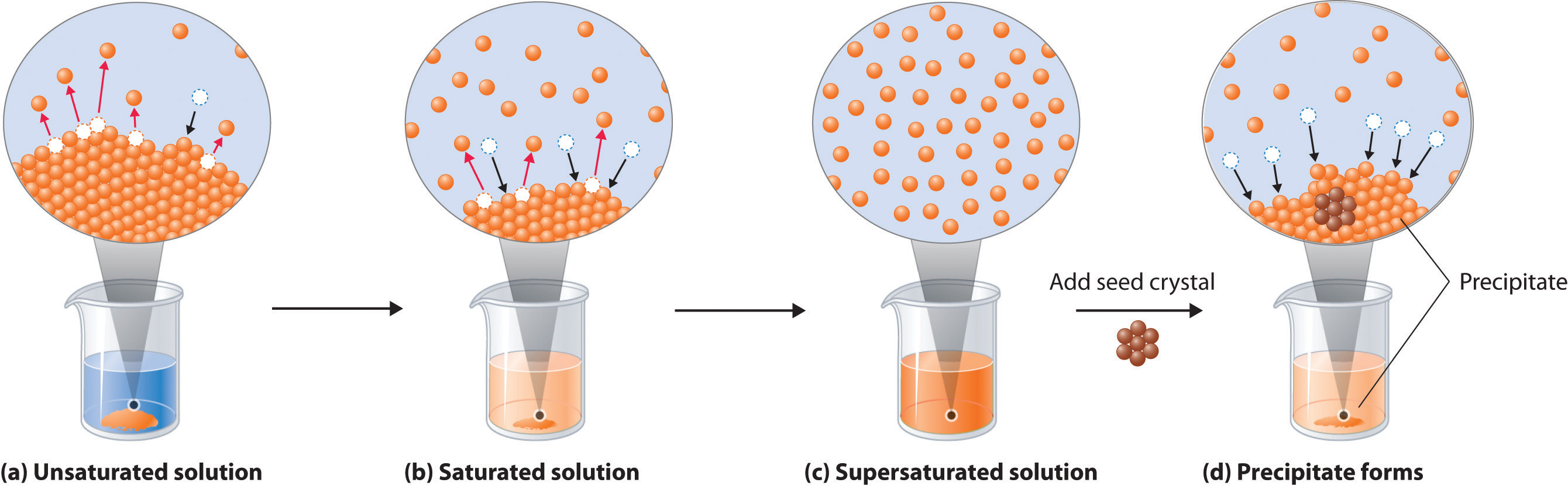



Chapter 9 2 Solubility And Structure Chemistry Libretexts




A Types Of Mixtures Pdf Free Download



What Is The Difference Between Solute Solvent And Disperse Phase And Dispersion Mediam Quora
コメント
コメントを投稿